Bivalent vaccine
The current COVID-19 vaccines. New bivalent COVID vaccine may not be more protective but its still recommended.

Covid 19 Bivalent Boosters Johns Hopkins Bloomberg School Of Public Health
This helps to create a broader immune.
. The bivalent booster is the most recent version of the COVID-19 vaccine. The Pfizer-BioNTech vaccine is authorized for use as a booster dose in individuals 12 years and older and the Moderna bivalent vaccine as a booster dose in. Simply put a bivalent vaccine is a type of vaccine that protects against a combination of two or more coronavirus strains.
The bivalent COVID-19 vaccines include a component of the original virus strain to provide broad protection against COVID-19 and a component of the omicron variant to provide. The results showed that a booster of the bivalent vaccine triggers a strong immune response against both the original Wuhan strain and omicron BA1. Isaac Bogoch expects we could be running three simultaneous vaccine programs.
The bivalent is designed to offer better protection against the more recent Omicron variants. The vaccine does not require. They compared virus neutralization by sera collected from individuals vaccinated with three doses of the original monovalent vaccines and a fourth dose of the bivalent vaccine.
The bivalent vaccine which Moderna has said it hopes will be authorized for use in the United States this fall is designed to target both the original omicron variant and the original. Data collected by the FDA for earlier bivalent COVID-19 booster vaccines suggests that these shots successfully provided immunogenicity a boost to your immunity and elicited. The safety of a single booster dose of the Moderna COVID-19 Vaccine Bivalent for individuals 18 years of age and older is supported by safety data from a clinical study which.
This can means two different viruses or two variations of one virus. A booster program a. A bivalent vaccine elicits an immune response against two different antigens.
Early trial results found the bivalent vaccine which is designed to target both the omicron variant and the original coronavirus strain in a single shot led to an eightfold. The Ministry of Health MOH will be bringing forward the administration of the bivalent ModernaSpikevax vaccine from 17 October 2022 which was previously announced. An updated version of the COVID-19 vaccine made by Moderna that targets two coronavirus variants known as a bivalent vaccine has today been.
These bivalent vaccines help to create a broader immune response and improve the strength and duration of protection against circulating variants. A bivalent vaccine is a Covid-19 vaccine booster that targets both the original strain of Covid-19 and the Omicron variant offering broad protection against Covid-19 and better. Health Canada has authorized another Moderna bivalent COVID-19 booster vaccine for those 18 years and older to tackle the Omicron BA4 and BA5 subvariants of COVID-19 two.
If bivalent shots are ready for fall infectious disease expert Dr. The FDA authorized bivalent formulations of the Moderna and Pfizer-BioNTech COVID-19 vaccines for use as a single booster dose at least two months after completing. Both are approved as a single booster dose at least 2 months following.
Vaccination with the bivalent Covid-19 mRNA booster vaccines has began. Health Canada has approved Pfizers new bivalent COVID-19 vaccine that contains mRNA from both the original SARS-CoV-2 virus and the Omicron BA4 and BA5 variants. The Moderna bivalent vaccine is presented as a blue-capped multi-dose vial 100 mcgmL containing either five 05mL doses or ten 05mL doses.
It contains both the original vaccine strain of the virus and a strain derived from the BA5 omicron variant. Kaiser nurse Marilyn Antonio left gives a Moderna booster shot to Ted Naifeh right from San. The bivalent Pfizer vaccine is authorized as a single booster dose in individuals 12 years of age and older.
Some Who Rushed To Covid 19 Vaccine Hold Off On Boosters Wsj

A Bivalent Omicron Containing Booster Vaccine Against Covid 19 Nejm
Omicron Ba 4 Ba 5 Bivalent Mrna Vaccine Booster Elicits Similar Neutralizing Antibody Responses To Monovalent Vaccine

Covid 19 Vaccination Information Arkansas Department Of Health

Covid 19 Vaccine Faq S Licking County Health Department
Vaccines Boosters Fishers In Official Website

Pfizer And Biontech Announce Positive Results Bivalent Booster Trials Healthcare Purchasing News

Moderna S Bivalent Booster Becomes First Authorized Omicron Vaccine

Moderna Says Its New Vaccine Booster Shows Superior Response To Omicron Npr
Bivalent Covid 19 Booster Vaccine Receives Mhra Approval Hospital Pharmacy Europehospital Pharmacy Europe

Should I Time My Covid Booster Medpage Today

Moderna On Twitter The Government Of Canada Has Exercised Its Option To Purchase An Additional 4 5 Million Doses Of An Omicron Containing Bivalent Vaccine Booster Candidate In Addition To Moving Forward The Delivery

Covid 19 Bivalent Vaccine Boosters Fda
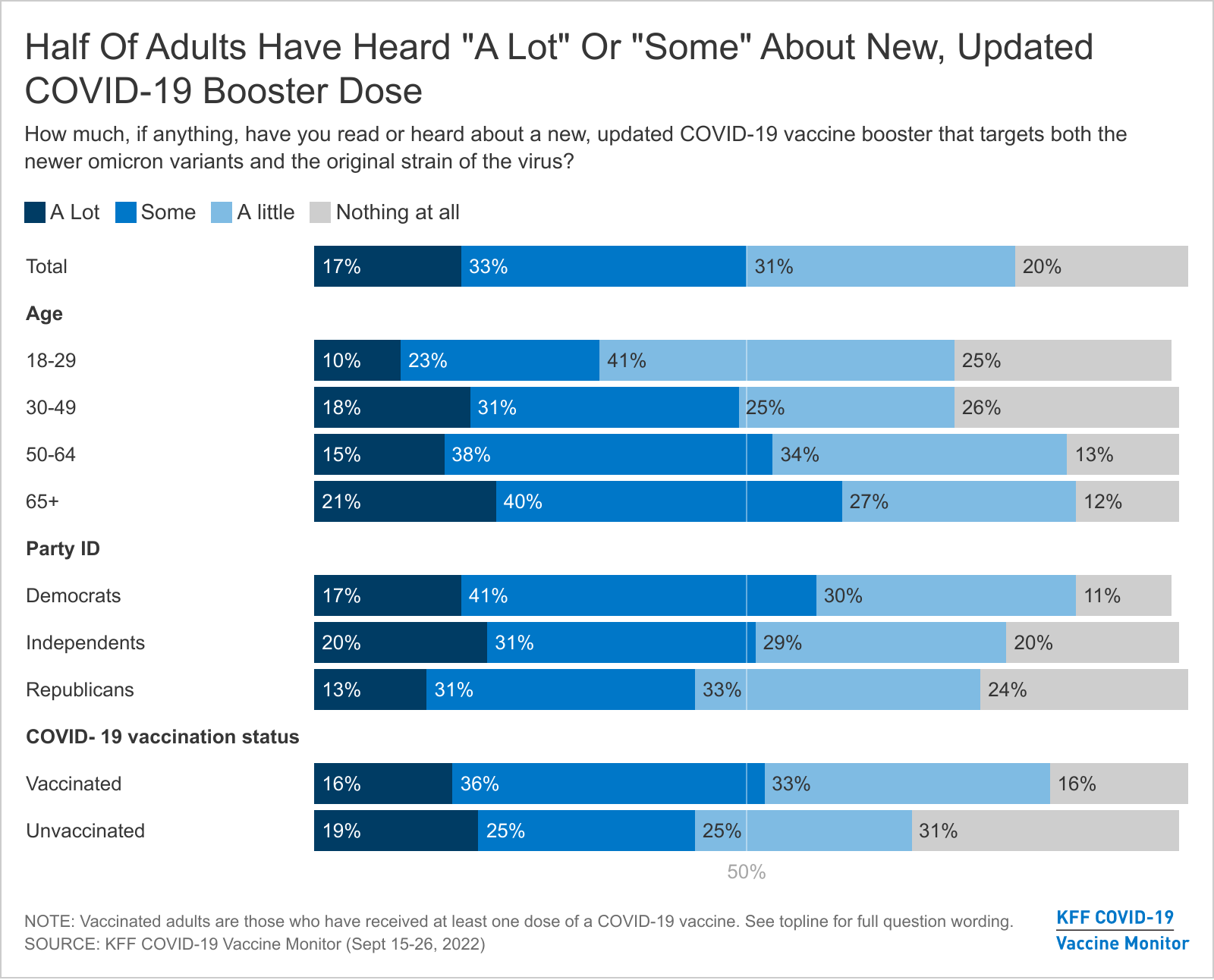
Half Of Public Has Heard Little Or Nothing About The New Covid 19 Booster Aimed At Omicron Many Don T Know If The Cdc Recommends That They Get The New Booster Kff

Covid 19 Bivalent Booster New Health Mil
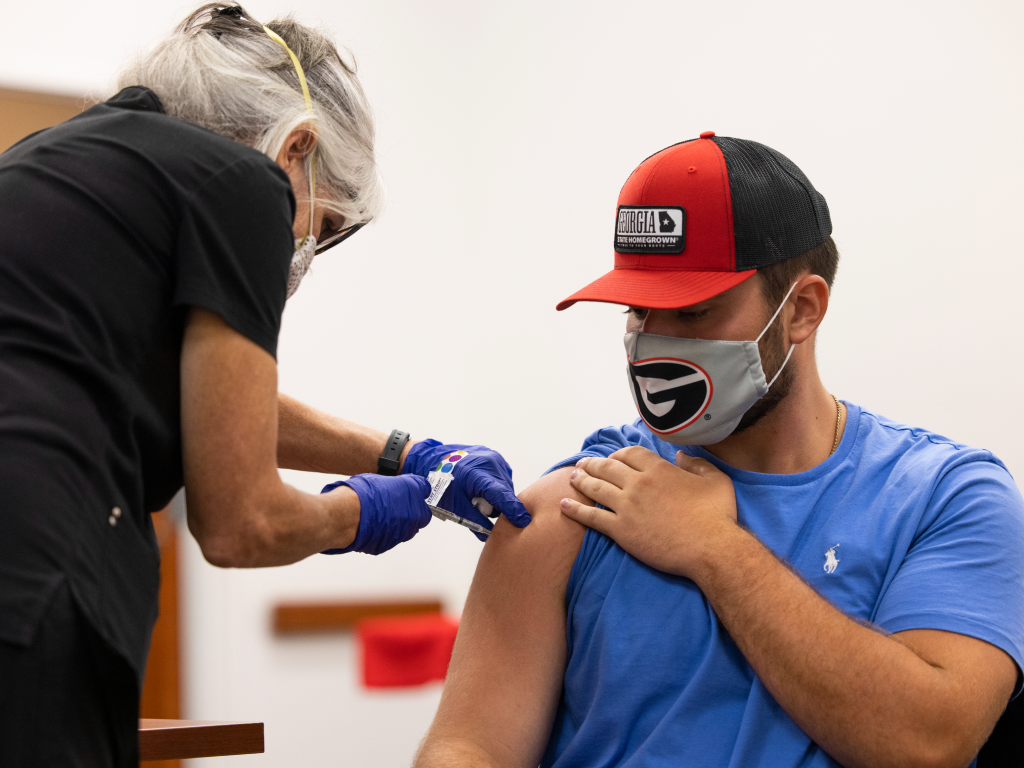
Covid 19 Bivalent Vaccine Available At Uhc Uga Student Affairs

Why And When To Get A Bivalent Covid 19 Booster Johns Hopkins Bloomberg School Of Public Health
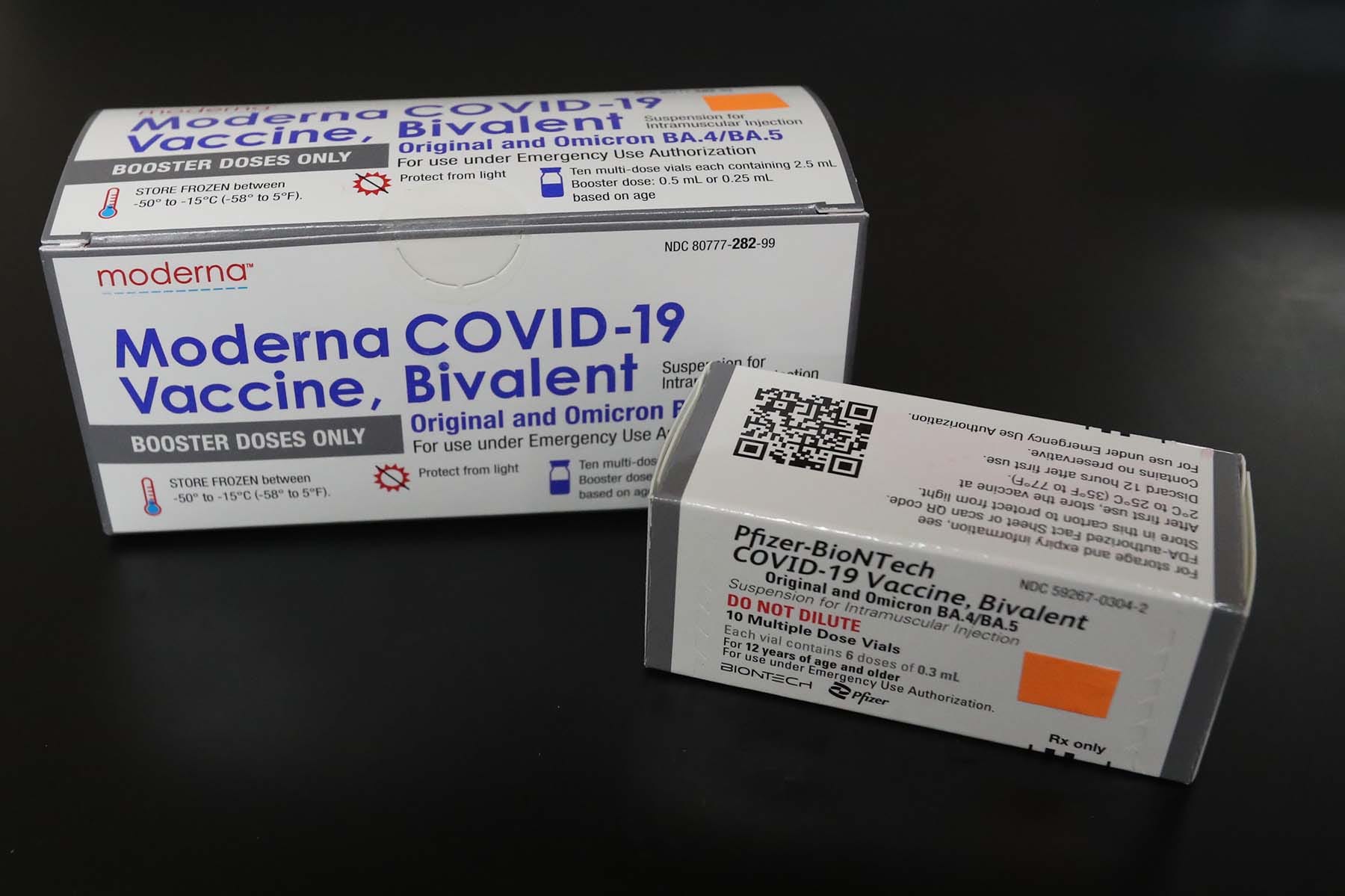
Updated Covid 19 Bivalent Booster Shots Now Available In Coachella Valley
What You Need To Know About Bivalent Boosters For Covid 19 Hub