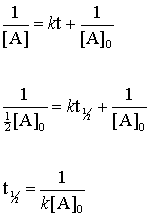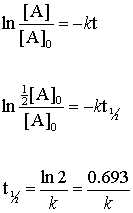half life formula for zero order reaction
The half-life equation for a zero-order reaction is latext_frac12fracA_02klatex. The half-life of a reaction t12 is the time required for one-half of a given amount of reactant to be consumed.

Zero Order Reactions Video Kinetics Khan Academy
Determining a half life.
. Equations for Half Lives. The half-life of a reaction describes the time needed for half of the reactant s to be depleted which is the same as the half-life involved in nuclear decay a first-order reaction. From the above formula the half-life of the zero order kinetics depends on the initial concentration of the reactant.
The timescale in which there is a 50 reduction in the initial population is referred to as half-life. T12 A 02K. Half-life is denoted by the symbol t 12.
The half-life of a reaction t 12 is the time required for one-half of a given amount of reactant to be consumed. ½ A A 0 kt 12. The Half-Life of Zero Order Reaction calculator computes the half-life in nuclear decay for a zero order reaction.
Frac 1 A_02 frac 1 A_0 kt_ 12 frac 1 A_02 - frac 1 A_0 kt_ 12. T ½ 1 k A o Top. Substituting these terms into the rearranged integrated rate law and simplifying yields the equation for half-life.
A A 0 - kt. The rate constant k will have units of concentrationtime such as Ms due to. We identified it from obedient source.
In each succeeding half-life half of the remaining. In each succeeding half-life half of the remaining concentration of the reactant is consumed. Given below is the half-life of a zero-order reaction.
From the integral form we have the following equation. The half-life of a Zero-th order reaction is t A0 2kHere I derive this from the Integrated Rate LawAsk me questions. As for other reaction orders an equation for zero-order.
Graphical relations and half lives. Rate k C12H22O11 Half-Life of a reaction t12. The rate constant for a Zero-order reaction rate of constant k.
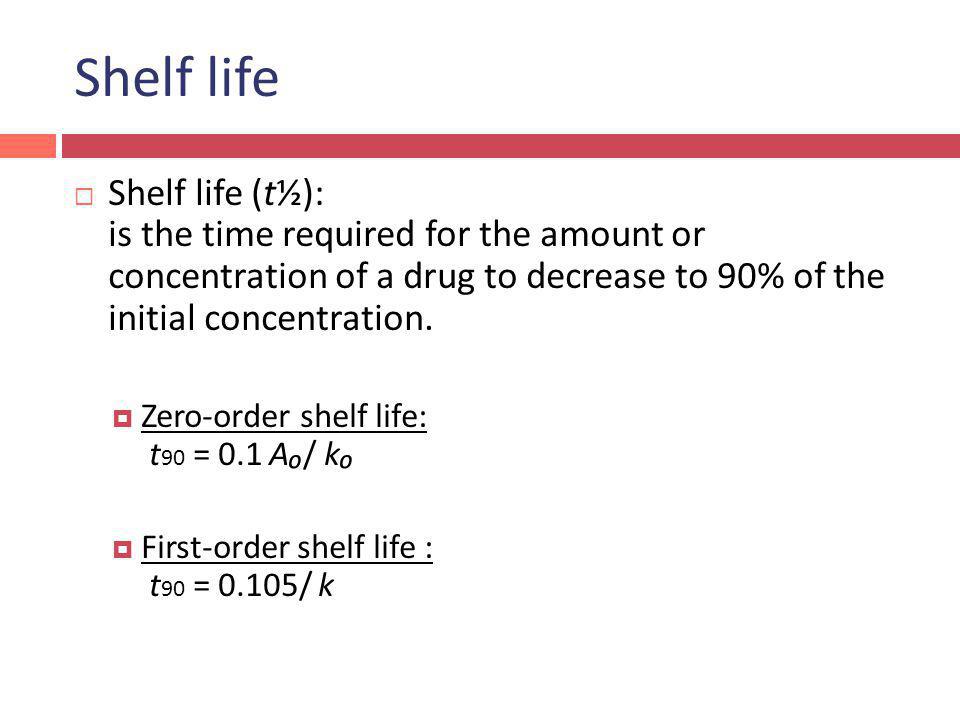
Converting a half life to a rate constant. The mathematical expression that can be employed to determine the half-life for a zero-order reaction is t 12 R 0 2k. The first-order reaction half-life equation is given by k 2303 t l o g R 0 R From the definition of the half-life of a first-order reaction at t t12 and R R 02.
The Half-Life of a Reaction. When t t ½ that is the half-life of the reaction completed the concentration of the reactant A A2. T ½ A o 2k For a first order reaction A products rate kA.
Half-Life of a Zero-Order Reaction. The decomposition of NH 3 on a tungsten W surface is a zero-order reaction whereas on a quartz SiO 2 surface the reaction is first order. Here are a number of highest rated Zero Order Half Life Equation pictures on internet.
For the first-order reaction the half-life is defined as t 12 0693k. For a zero order reaction A products rate k. The half-life of a first-order reaction is given as t 12 0693k.
Now we have the following equation and can solve for eqt_ 12 eq. T1 2 A0 2k t. And for the second-order reaction the formula for the.
The half-life of the reaction is denoted by t 12 and is expressed in seconds. Using the decomposition of hydrogen peroxide see this lesson as an example we find that during the first half. Half-Life of a Zero Order Reaction.
Therefore A2 k 0 t ½ or t ½ A2k. For a zero-order reaction the half-life is given by. For a first order reaction t½ 0693 k and for a second order reaction t½ 1 k Ao.
For a zero order reaction the formula is t½ Ao 2k. For first order reaction we know that k 1t. The rate constant for the reaction can be determined from the slope of the line which is equal to -k.
T ½ 0693 k For a second order reaction 2A products or A B products when A B rate kA 2. Its submitted by dealing out in the best field. Term half-lifeThe time required for a quantity to fall to half its value as measured at the beginning of the time period.
Determine the half-life of a zero order react. Half-life or t½ is the time that elapses before the concentration of a reactant is reduced to half its initial. Remember the half-life of a reaction changes with the order of the reaction.
T_frac12 lnfracA_0frac12A_0times frac1k t_frac12 ln2times frac1k. Zero Order Half Life Equation - 16 images - half life deranged physiology zero order reaction and its half life chemical kinetics. Half life means 50 percent of reactants disappear in that time interval.
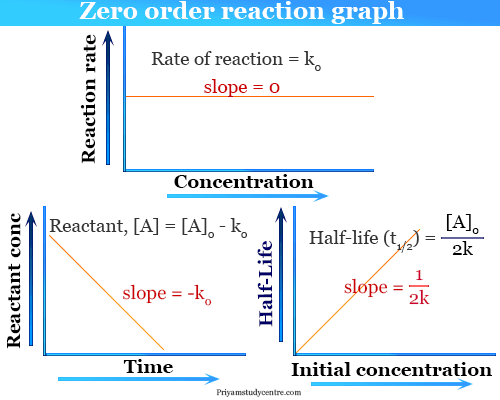
T 12 R 02k From the above relation we can say the Half-Life of a zero-order reaction is directly proportional to the initial concentration of the reactants and inversely proportional to the rate constantt 12 R 02k. Because this equation has the form y mx b a plot of the concentration of A as a function of time yields a straight line. 5 rows Zero-Order Reactions.
Replace t with half-life t 12. Half life in zero order reaction. The formula for half-life in chemistry depends on the order of the reaction.
It is to be noted that the half-life of a zero-order reaction is determined by the initial concentration and rate constant. The integrated rate law for the zero-order reaction A products is A_t -kt A_0.

Which Of The Following Statements Are Corrects

Kinetics Integrated Rate Law And Half Life Expression For General Nth Order Reaction N 1 Youtube

Half Life Expressions Chemistnate
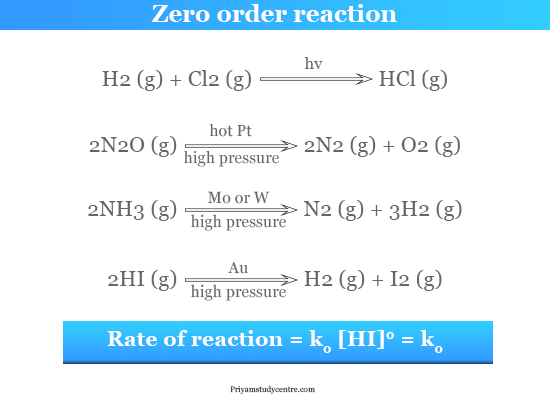
Zero Order Reaction Definition Examples Formula

Half Life Expressions Chemistnate

Derive The Integrated Half Life Equation For Zero Order Reaction Chemistry Chemical Kinetics 12889537 Meritnation Com

Integrated Rate Laws Chemistry For Majors

Half Life Of Zero Th 0th Order Reaction Derivation Youtube
Half Life Of A First Order Reaction Video Khan Academy

Kinetics Order Of Reactions Ppt Video Online Download

Half Life Expressions Chemistnate
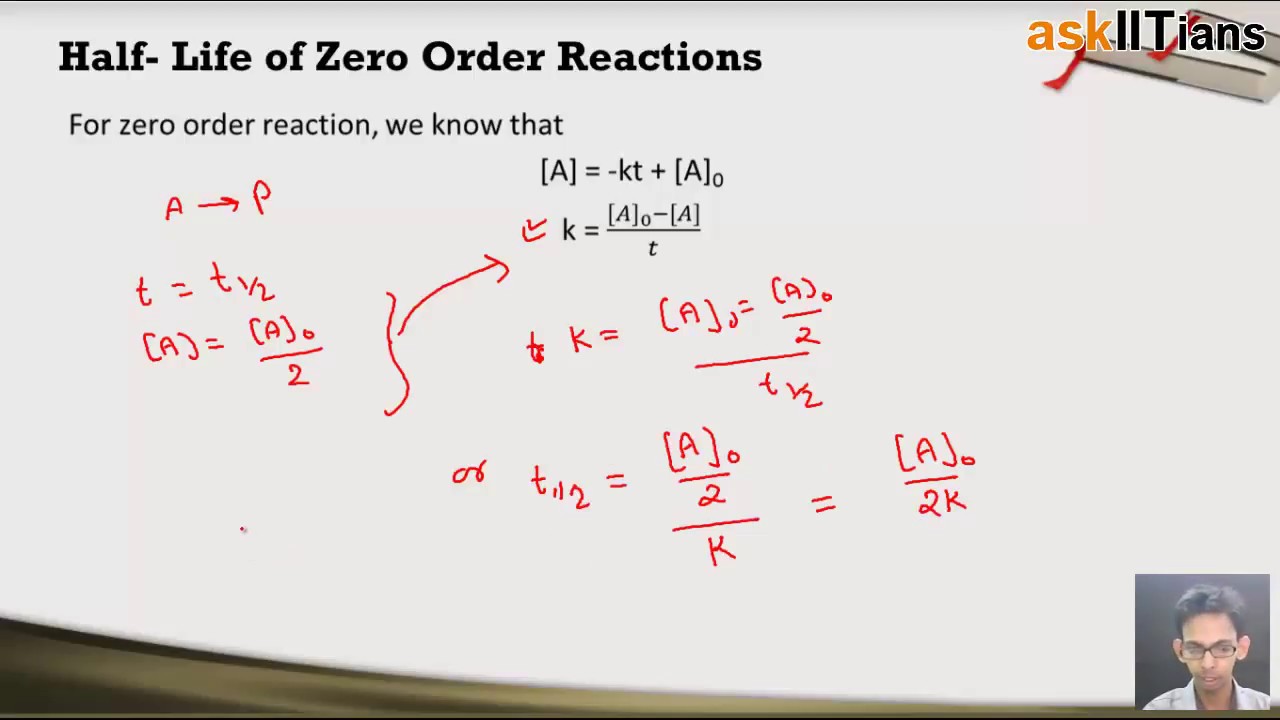
Zero Order Reactions Chemistry Class 12 Iit Jee Main Advanced Neet Aipmt Askiitians Youtube